The Australian Teletrial Program (ATP) is supported by funding from the Australian Government under the Medical Research Future Fund. The Program has been funded $75.2 million over five years to bring clinical trials closer to home for patients in Queensland, Victoria, Tasmania, South Australia, Western Australia and the Northern Territory.
ACTA's role
ACTA have partnered with the ATP to help in the development of Teletrials as a way to bring safe, cost effective and quality trials closer to home for many Australians. The project will deliver capacity building and education for Clinical Trial Networks and Coordinating Centres, to help plan and run Teletrials across regional, rural and remote sites in Australia and across all therapeutic areas.
Contact ACTA
Email: info@acta.au
Contact your local ATP Regional Clinical Trial Coordinating Centre (RCCC)
QLD RCCC QRCCC@health.qld.gov.au
ATP-SA ATP-SA@sa.gov.au
ATP-TAS ATP-TAS@health.tas.gov.au
ATP-NT ATP-NT@nt.gov.au
ATP-VIC RCCC@health.vic.gov.au
WA RCCC WARCCC@health.wa.gov.au
RESOURCES
ACTA has been working with the Australian Teletrial Program (ATP) to explore how Clinical Trial Networks and Coordinating Trial Centres have used the teletrial approach in their trials. We have identified several examples from our networks.
View the Teletrial web resource
This web resource is not intended to be a comprehensive how-to-guide but rather to promote a community of practice. That is, by sharing experiences we can collectively work toward enhancing access to trials for all Australians.
Teletrial Myth Busting and Information Update
Teletrials are where the coordinating site (Primary) connects with local sites (Satellite) to build capacity and capability for clinical trials across regional, rural and remote Australia.
Harmonisation of national policy and implementation tools
These tools are aimed at reforming what has been a long and arduous process for progressing through HREC and Research Governance. Key points of difference and the road from Protocol development through to patient recruitment
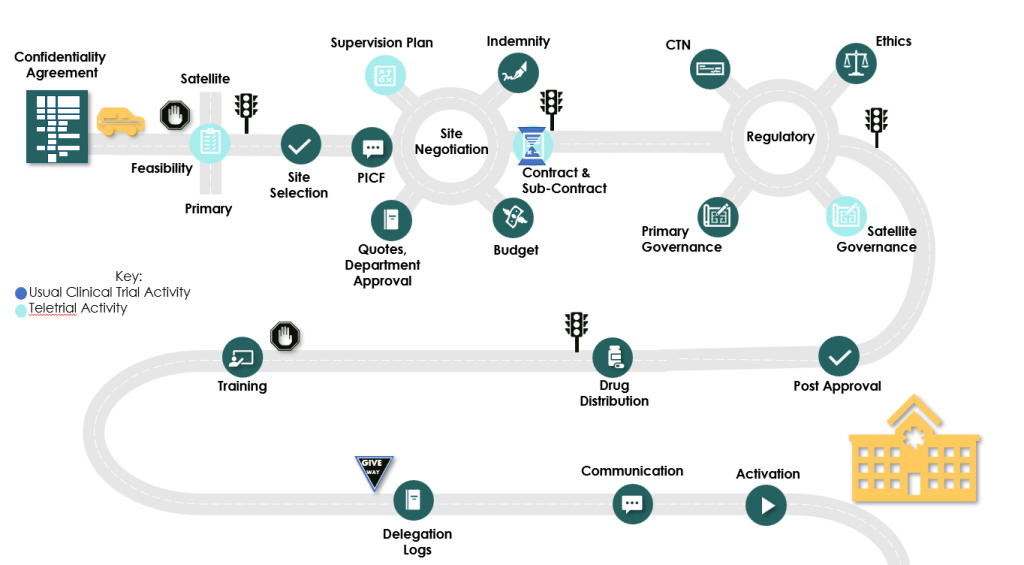
Teletrial Supervision Plan
The Teletrial Supervision Plan is a key document for the implementation of Teletrials
As the Teletrial model has expanded across jurisdictions, specialties, and interventions, multiple plans started to be used. This resulted in duplication of resources to recreate plans, different information being collected and inconsistent reporting for Sponsors.
National standardisation of documentation was identified as a key priority for national harmonisation and implementation of Teletrials across jurisdictional boundaries.
Collaborating with the Alfred Trial Hub, VCCC Alliance and the New South Wales Ministry of Health, the ATP facilitated an extensive review of the Teletrial Supervision Plan to achieve a nationally accepted document.
The revised Supervision Plan has had input from various stakeholders who manage and undertake Clinical Trials and Teletrials.
This included Research Governance Offices, Certified Human Research Ethics Committees, and Clinical Trial Sites with and without teletrial experience.
The Supervision Plan template is now available on the Australian Teletrial Program (ATP) website: Resources – Australian Teletrial Program.
New educational videos will be available soon on the ATP website to assist you to learn more about and complete a Supervision Plans.
How can we improve equity of access to clinical trials for patients who live in regional, rural and remote areas?
The Australian Teletrial Program is an innovative health equity program to improve access to clinical trials for patients closer to where they live, and makes clinical trials for new novel drugs, technologies, and models of care more accessible.
To date the program has been focused on setting up the infrastructure required to make teletrials available. There is now a fully operational Regional Clinical Trial Coordinating Centre (RCCC) in each participating jurisdiction: the Northern Territory, Queensland, South Australia, Tasmania, Western Australia and Victoria.
The teams are working hard to bring clinical trials closer to home for patients across specialties including renal, neurology, sexual health, mental health and palliative care. They are connecting clinical trial sites, and helping to build local capacity and capability, using the proven Teletrial model.
ACTA – ATP Webinar: Avoiding Groundhog Day
This informative webinar, held on 3 February 2025, featured insights from members of the ATP RGO Reform Working Group, who are leaders in research governance and research office operations.
The principles discussed are relevant to all clinical trial stakeholders including investigators, site staff, sponsor and research governance offices.
Speakers:
- Dr Shyamsundar Muthuramalingam – Consumer Advocate and Consumer Member, Clinical and Consumer Advisory Committee, ATP
- Alexandra Robertson – Director Research Operations, Perth Children’s Hospital (Child and Adolescent Health Services)
- Clare McKay – Acting Director, Clinical Innovation & Research, NT Health
- Sonia Hancock – Chair, Metro South HREC, Metro South Health Research, Queensland
- Nadine Herren – Clinical Trial Manager, ATP WA Regional Clinical Trial Coordinating Centre (WA RCCC), WA Country Health Service
Facilitated by: Prof Nik Zeps, Monash University Faculty of Medicine, Nursing and Health Sciences.
Watch the replay from 3 February 2025:
ACTA Webinar: The Australian Teletrial Experience: Tales from the Frontline
This webinar, held on 21 November 2023, featured speakers who shared their experiences and insights on the challenges, hurdles, and successes of running Teletrials within clinical trial networks.
Speakers included:
-
- Gabrielle Byars, Chief Executive Officer – Melanoma and Skin Cancer Trials (MASC)
-
- Naomi Sprigg, Clinical Operations Manager – Australasian Leukaemia & Lymphoma Group (ALLG)
-
- Jonathon Lennon, Clinical Trial Operations Coordinator – NHMRC CTC
-
- Kelly Foran, RRR consumer Representative

