Economic evaluation of investigator-initiated clinical trials conducted by networks
In July 2017, ACTA and the Australian Commission on Safety and Quality in Health Care published a landmark report detailing a series of case studies used to evaluate the health and economic benefits of trials conducted by clinical trials networks.
The three Australian Clinical Trial Networks evaluated in the report were the Australasian Stroke Trials Network (ASTN), Interdisciplinary Maternal Perinatal Australasian Collaborative Trials (IMPACT) Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS CTG). These three well-established networks have collectively overseen more than 460 individual trials.
The evaluation followed a six-stage process to:
- Understand the potential impact of the trials on clinical practice and/or policy
- Identify the number of people affected if the trial findings were implemented
- Calculate the benefit of trial findings on ongoing patient health outcomes
- Calculate the benefit of trial findings on ongoing direct health service costs
- Measure the benefits against clinical trial and network costs
-
Undertake sensitivity analyses to investigate what would happen to the results if assumptions changed.
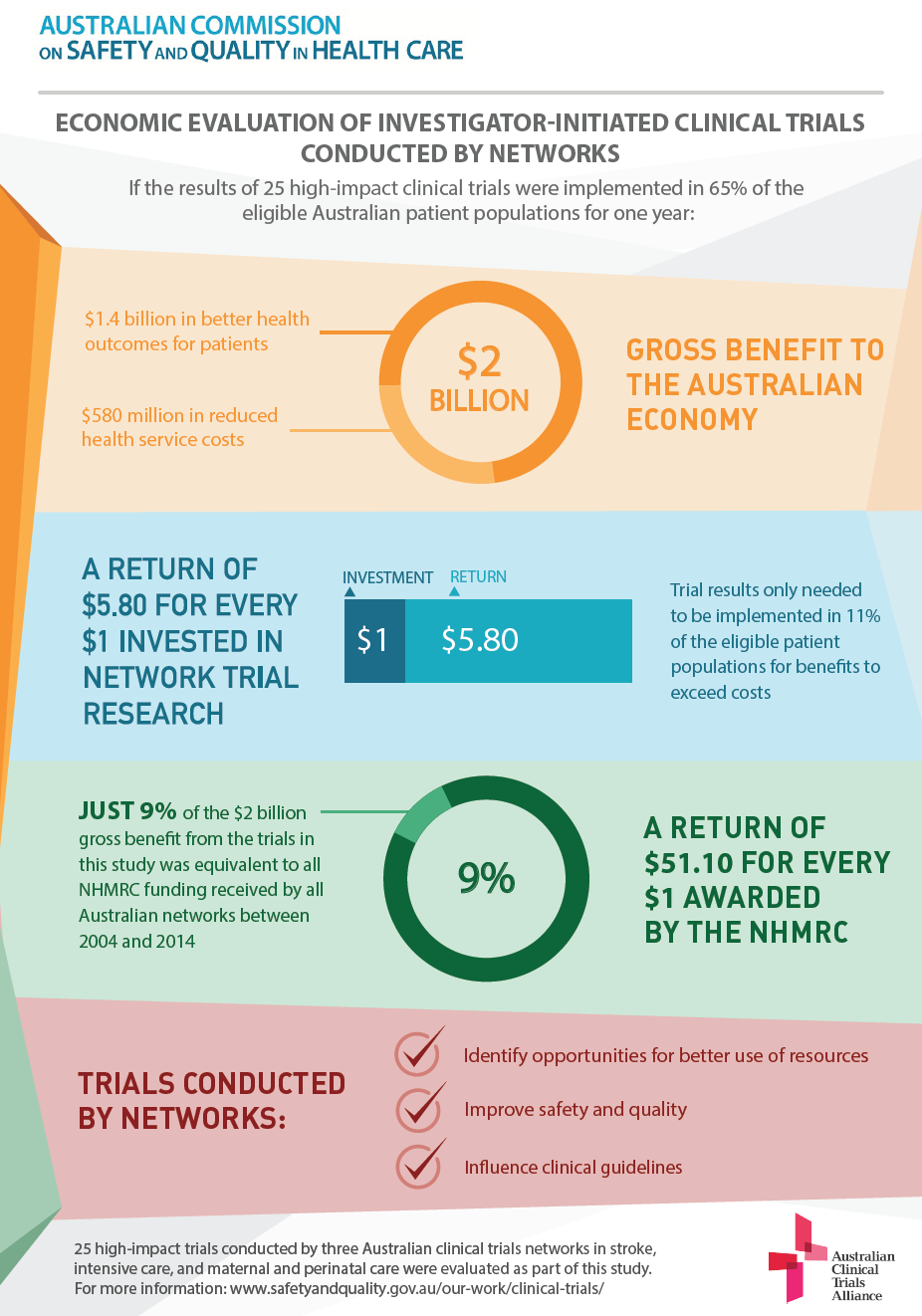
In total, 25 high-impact clinical trials were evaluated across the three networks. If the results of these trials were implemented in 65% of the eligible Australian patient populations for one year:
-
The gross benefit would be approximately $2 billion (2014 dollars) measured through better health outcomes and reduced health service costs
- Reductions in health service costs would account for 30% ($580 million) of the gross benefit, and this alone would exceed the total costs for the three networks and all of their research activity from 2004 to 2014.
The report also finds:
- The overall consolidated benefit-to-cost ratio for the networks is 5.8:1, or a return of $5.80 for every $1 invested
- The results of the 25 trials only needed to be implemented in 11% of the eligible patient populations for benefits to exceed costs
- For every $1 awarded in National Health and Medical Research Council (NHMRC) grants to the 25 trials, a return of $51.10 was achieved
- Just 9% of the $2 billion gross benefit from the trials in this study was equivalent to all NHMRC funding received by all Australian networks between 2004 and 2014.
Report on the Activities and Achievements of Clinical Trials Networks in Australia 2004–2014
ACTA's major report on the activities and achievements of Clinical Trials Networks in Australia has been officially published. The report was commissioned by the National Health and Medical Research Council (NHMRC) and undertaken by ACTA. The report provides the first detailed snapshot of clinical trials network activity in Australia over the decade 2004 – 2014.
Thirty-four networks took part in the study which has shown that Australian clinical trials networks have together completed and published or initiated more than 1,000 individual studies, involving more than 1 million participants and representing more than $1 billion in total research funding over the last decade.
This is a huge catalogue of investigator-initiated or "public good" clinical trials and large observational studies (more than 90% of studies were non-commercial studies) which includes more than 100 high-impact studies that have, or are likely to, change practice and policy both in Australia and around the world.
Australia has long been regarded as a world leader in the design and conduct of large phase 2, 3 and 4 clinical trials within a number of individual clinical disciplines. However, until now much of what is known about our national clinical trials capacity and expertise has been limited to the commercial trials sector.
This report will provide Government with a much better understanding of how important clinical questions are addressed through public good trials that are designed and conducted by networks that incorporate many thousands of practising clinicians and researchers across Australia – many of whom conduct research over and above their regular clinical work.
ACTA acknowledge with much appreciation all the networks that took part in this landmark project, and the tireless efforts of all members of the Steering Committee and Project Team and the support received from the NHMRC Clinical Trials Section.
A copy of the report is available here.
For further information about the Profiling Networks Project contact Simone Yendle, General Manager, +61 9903 0088